DrugCard
Links
Description
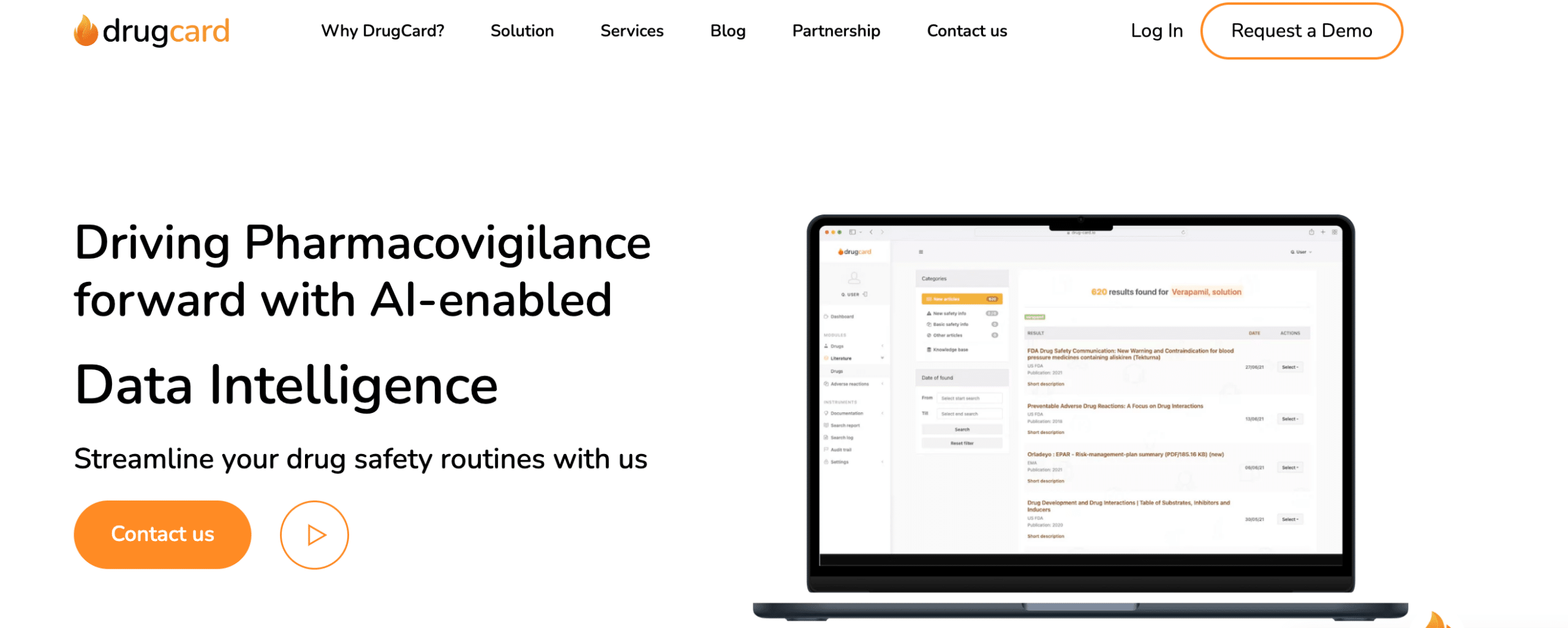
DrugCard is a literature monitoring platform that helps pharmacovigilance professionals automate local literature screening and turn intelligence into actions, from monitoring to management and reporting. It uses artificial intelligence to identify and extract relevant information from medical literature, such as clinical trials, case reports, and regulatory filings. This information can then be used to identify potential safety risks, monitor the efficacy of drugs, and support regulatory decision-making.
DrugCard offers a variety of features, including:
- Automated literature screening: DrugCard automatically scans large volumes of medical literature for relevant information. This saves pharmacovigilance professionals time and allows them to focus on other tasks.
- AI-powered data extraction: DrugCard uses AI to extract relevant information from medical literature, such as the name of the drug, the patient's demographics, and the adverse event. This information is then presented in a clear and concise format, making it easy for pharmacovigilance professionals to review.
- Collaboration tools: DrugCard allows pharmacovigilance professionals to collaborate on literature screening and case management. This helps to improve efficiency and communication.
- Reporting tools: DrugCard provides a variety of reporting tools that pharmacovigilance professionals can use to generate reports on their findings. This can help to support regulatory decision-making and improve patient safety.
DrugCard can be used by pharmacovigilance professionals at pharmaceutical companies, CROs, and regulatory agencies. It is a particularly good fit for organizations that have a high volume of literature to screen or that are looking to automate their pharmacovigilance process.
Here are some of the use cases for DrugCard:
- A pharmaceutical company is using DrugCard to monitor the safety of their new drug. DrugCard is helping the company to identify potential safety risks and to track the incidence of adverse events.
- A CRO is using DrugCard to support the pharmacovigilance activities of their clients. DrugCard is helping the CRO to screen medical literature for relevant information and to generate reports on their findings.
- A regulatory agency is using DrugCard to monitor the safety of drugs on the market. DrugCard is helping the agency to identify potential safety risks and to take appropriate regulatory action.